The Importance of the Intermediaries of the
Citric Acid Cycle for Bioregulatory Medicine
The citric acid or tricarboxylic acid cycle (TCA cycle) localized in the mitochondria and the interconnected electron transport chain (ETC) lead to the formation of ATP, the most important energy source of the cell.
The TCA cycle is the shared final stage for the dismantling of all nutrients and is therefore the actual life preserving turntable of the metabolism.
There all components of the tissues (carbohydrates, proteins and lipids) are first converted enzymatically into Acetyl-CoA (activated acetic acid). The first substrate or intermediary in the cycle is citrate. The further steps (⇒ citrate ⇒ cis-aconitate ⇒ isocitrate ⇒ ketoglutarate ⇒ succinate ⇒ fumarate ⇒ malate ⇒ oxaloacetate ⇒ citrate) contribute to the production of ATP (the arrows indicate the corresponding transforming enzymes): The TCA cycle with the linked ETC is the most productive cellular source for indirect production of ATP. The enzymes are regulated and steered by regularization mechanisms like feedback, end product or competitive inhibition. Possibly appearing intracellular congestions, blockages, shifts or substrate deficiencies within the flow system can then lead in turn to the development of cell destruction. Reckeweg (1993) classified the impregnations- and degenerations-phase into a six phase table developed by him. As a consequence he developed from this a “therapy with intermediary catalysts” (Reckeweg 1993, overview by Schmid et al. 1996). Reckeweg (1993) designated the homeopathic intermediaries of the TCA cycle as catalysts of Group A. As an effective mechanism it was simply referred to as the TCA cycle, because the empirical experiences spoke on behalf of this general assessment. The intermediaries can be used as an Injeel individually or as a combination with all illnesses appearing on the right from the biological section. (Heine et al. 2002).
With the help of a data base study (MEDLINE, Pub Med and DIMDI starting from 1965) with the keywords “citric acid”, “cycle”, “intermediates” and “receptors”, the goal of this work is to find indications of the extracellular cell membrane receptors for the intermediaries of the TCA cycle arrived into the extracellular space and the interconnected signal transduction. For the time period 1965 through 2011 five relevant works were found.
Intermediaries of the Citric Acid Cycle in the Blood and Tissues
The intermediaries of the TCA cycle from damaged cells in the blood and tissues are known for a long time (Goldberg et al. 1966, Martin et al. 1989, Herbert 2004, Correa et al. 2007). The phenomenon was more exactly examined by He et al. (2004) and it was shown that their plasma concentrations lie in the micromolecular area. This is comparable to the intermediaries of the TCA cycle used in anti-homotoxic medicine as therapeutics (Heine et al. 2002).
There are three forms of cell destruction that can arrive into the blood stream and the tissues through the intermediaries of the TCA cycle from the mitochondria:
Apoptosis
Apoptosis is also called programmed or physiological cell death (overview by Hotchkiss et al. 2009). Without this mechanism an 80 year old would accumulate approximately two tonnes of bone marrow and the bowel would have a length of approximately 16 km (Melino 2001). The trigger for apoptosis is any form of a longer lasting cell stress (DNA disturbances, x-ray radiation, oxygen radicals among other things; overview by Hotchkiss et al. 2009). At the same time, this makes a distinction between an extrinsic activation over the “death receptor” in the cell surface and an intrinsic [activation] over the mitochondria (overview by Hotchkiss et al. 2009).
The extrinsic path can be followed by many cell types especially CD4+T cells. Nevertheless, the so called Fas-signal path is triggered. The Fas-protein (Apo-1, CD95) includes a “death domain” and binds to the Fas ligand (Fas l.). These represent members of the TNF receptor family (Wajant 2002).
This has particular importance for the latent readiness of the extracellular matrix (ECM) toward inflammation.
As a result their proinflammatory readiness is substantially regulated by apoptosis, by which important contributions are made to the development, homeostasis, tumor observation and function of the immune system (Wajant 2002, Heine 2006).
After repeated stimulation (e.g. to CD4+T cells) by autoantigens the Fas are also expressed as Fas l. The connection in Fas triggers apoptosis over the activation of cytoplasmic caspase (protease), at the same time caspase 8 has crucial importance (Fig. 1). With this the cell membrane is not triggered, but it constricts vesicles (“blebs”), which are triggered in the extracellular space and sets free their biologically active contents, among other things the intermediaries of the TCA cycle (Fig. 1). On that occasion the cells become smaller and are finally phagocytized by macrophages (Hotchkiss et al. 2009).
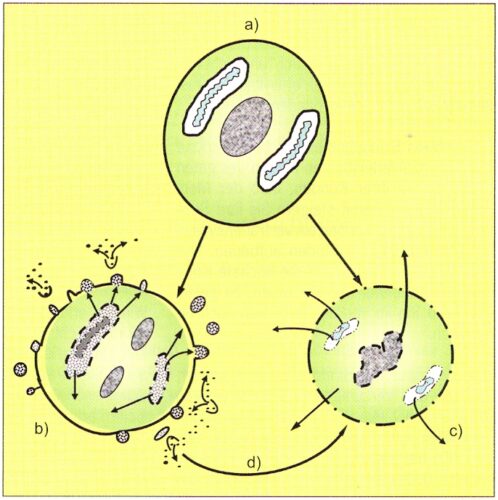
Figure 1: Diagram of the three forms of cell destruction: A normal cell (a) can perish through apopotosis (b), the physiological cell destruction, or accidentally through necrosis (c). The physiological leukocytolysis (d) represents a transition between apoptosis and necrosis.
The intrinsic path is triggered after intracellular stress by increased formation of stress proteins (heat shock proteins and other chaperones). Misfolded proteins which can no longer be brought into their functional structure by the heat shock proteins can also be formed through long term high demand of synthesis output of the endoplasmatic reticulums (ER) with respect to proteins and their secondary structure (e.g. the globulin produced by B cells or in the bordering intestinal lumen, only approximately 15 hours of “living” epithelial cells with their high output spectrum). They collect in the cytoplasma, are finally no longer reduced by the proteasomes and trigger apoptosis by “softening” the external mitochondrial membrane by release of cytochrome c and by activation of caspase 9 and 3 (overview by Hotchkiss et al. 2009).
Cell Necrosis
While the apoptotic cell shrinks without loss of the cell membrane by constriction of the vesicles (blebs), cell necrosis runs off under loss of integrity of the cell membrane. Fluid and ions stream into the cell by which it and its organelles swell and finally tear apart under emission of their cell contents, whereby the intermediaries of the TCA cycle are released by the declining mitochondria (Fig. 1).
Triggering of necrotic cell destruction is hypoxia, ischemia with rapid loss of ATP, high inflow of calcium ions and increased formation of oxygen radicals (overview by Heine 2006).
Physiological Leukocytolysis
A very significant way for release of the intermediaries of the TCA cycle into the blood and tissues is physiological leukocytolysis (PhL). This was known since Undritz (1941) and was more exactly investigated by Pischinger, Heine and Draczynski (overview by Heine 2006). Regarding the actual and situation-adapted PhL it reacted to deviations of the homeodynamic no matter what kind.
All biological medicine procedures stimulate PhL to support the organism in its control behavior (Heine 2006).
Nevertheless, it does not(!) concern aged cells, but rather physiological-lytic forms (Fig. 2). With an absolute number of 5,000 leucocytes per mm3, according to Pischinger (1975) 300 lysis forms are found per mm3. In approximately five liters of blood one to two billion leukocytes are thus found in dissolution at any time. This corresponds to about 1.2 million actually dissolved leukocytes per second (Pischinger 1975). As a result in each case up to a half gram of leukocyte ingredients should be released, to which also belong the intermediaries of the TCA cycle (Heine 2006). Besides, the neutrophil granulocytes stand in the center quantitatively with a 55 to 68% portion in the differential blood count and a life span of two to eight days.
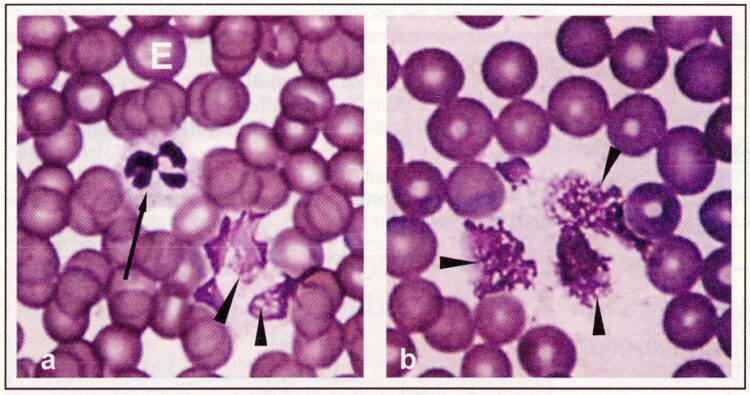
Figure 2: Physiological Leukocytolysis. Blood smear (person). (a) More normal neutrophil granulocyte (arrow) beside physiological lytic granulocytes (arrow heads). (b) Physiological lytic neutrophil granulocytes (arrow heads) in a patient with an influenza infection. The undamaged erythrocytes (E) show that it does not concern an artifact. H-E coloring, 1000 times enlargement (from: Dracynski 1998).
With the PhL there seems to be a middle pathway available between initial apoptosis and final necrotic cell destruction (Fig. 1). Then the turnover seems to occur. When the leukocytes go into apoptosis on the basis of cell stress, they form misfolded and aggregated proteins as well as increased oxygen radicals, by which the formation of Fas and Fas ligands are stimulated. Additionally a rapid loss of ATP takes place. RIP3 (protein kinase receptor interacting protein 3) is thereby activated, which steers the changeover from apoptosis into necrosis under the release of the intermediaries of the TCA cycle (Wajant 2002, Zhang et al. 2009).
Receptors of the Intermediaries of the TCA Cycle
The importance of local interstitial accumulation of intermediaries of the TCA cycle as a signal of ischemic organ damage or as indicators for the imbalance between energy supply and demand was already pointed out by Goldberg et al. (1966) and more recently by Correa et al. (2007) and in an overview by Herbert (2004).
It was first shown by He et al. (2004) that these have intermediary ligands for G protein coupled receptors (GPCR). GPCR apparently occur on all cell membranes (He et al. 2004). They are regulated by the cell respiration, the metabolic relationship as well as the renal excretion and retention (He et al. 2004).
He et al. (2004) could also prove that GPCR 91 represents the receptor for succinate, and GPCR 99 for a-ketoglutarate. Rubic et al. (2008) indicated that the connection of succinate in GPCR to dendritic cells produces their immune performance. Succinate has an effect on the GPCR 91 through mobilization of the intracellular calcium ions and induction of the cell migration and works together synergistically with the toll receptor ligands for production of proinflammatory cytokines. GPCR 91 is important as a sensor for immunological problems.
In general the G proteins are in connection with all important intracellular signal pathways (Fig. 3) (overview by Neves et al. 2002). Alternatively Pajor et al. (2010) was able to show in vitro that the intermediaries of the TCA cycle which are formed with information from the food in the intestine, can be passed carrier-mediated by the intestinal epithelial cells.
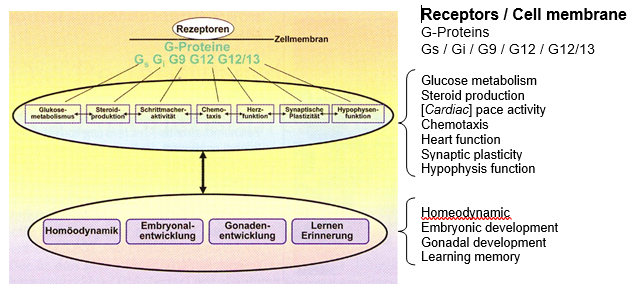
Figure 3: G protein coupled signal paths. Four families of G proteins are differentiated: Gs, Gi, Gq, G12/13. They are involved in the integration of all systemic functions (according to Neves et al. modified 2002). [See beside the diagram for an English translation of these terms.]
Construction and Function of the Heterotrimeric G Proteins
The guanosine triphosphate (GTP) binding protein (G protein) is on the inside of the cell membrane (Fig. 4). They usually represent an inactive heterotrimer (a-, b-, g-subunit), which is split after receptor activation (often a seven times membrane force-through receptor) into the subunits bg and a (Fig. 4). With the receptor activation the GTP bond occurs in the G protein. Thereby the split off a-subunit (Ga) an then initiate (Gs protein) or inhibit (Gi protein) the signal transduction chains across the adenylate cyclase to cAMP (Fig. 4).
Intermediaries of the TCA Cycle
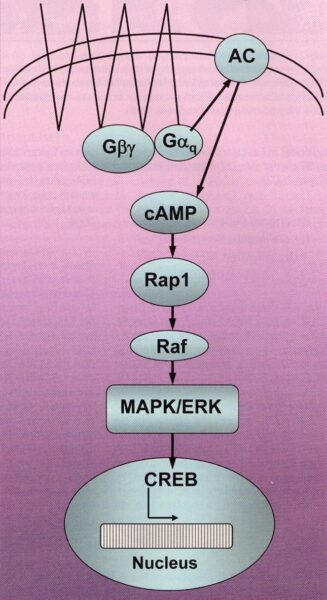
Figure 4: A deeply simplified diagram of a G protein coupled signal transduction cascade as an example of the Gs proteins. At the beginning is the binding of suitable molecules (e.g. the intermediaries of the citric acid cycle (TCA Cycle)) to a receptor (here a seven times force-through of the cell membrane) which binds on the inside of the cell membrane to a heterotrimeric guanine nucleotide (Gabg). By splitting off of the Gaq the adenylate cyclase is activated, by which ATP is converted to the second messenger substance cAMP. cAMP connects the obtained information with metabolic enzymes, by which the transcription factor CREB (cAMP response element binding protein) is activated and in the cell nucleus the corresponding gene is activated. MAPK/ERK (Mitogen activated protein kinase / extracellular signal regulated kinase); Raf (a MAP-kinase kinase); Rap1 (a “small” GTP-ase) (using an illustration from Neves et al. 2002).
Because the α subunit possesses a GTP-ase activity the signal transduction process is terminated by self activation over and over again after a short time. After splitting of the GTP by the a subunit into guanosine diphosphate (GDP) and phosphate (Pi), the a subunit can further reassociate with the bg subunit by which the receptor binding is once more available (overview by Stryer 1988).
Disturbances of the signal transduction at the level of the G proteins can be triggered by mutations and acquired modifications (e.g. by cholera or pertussis toxins).
The so called “small G proteins”, among others the ras gene family, show sequence homologies to the a subunits of the G proteins. Mutations of the ras proteins (“oncoproteins”) by long term activation can lead to uncontrolled growth and malignant cell degeneration. Ras-mutations are found in 10 to 15% of all human tumors, and they are often found especially (approximately 90%) in pancreas carcinoma (Farfel et al. 1999).
Importance of the G Protein for the Homeodynamic
Through a constant supply of ligands (e.g. through the intermediaries of the TCA cycle) for the GPCR a certain adaptation in catalysis occurs and a reorganization of the GTP ⇔ GDP reaction. This is to be evaluated as favorable because the signal transducing systems are geared to react to changes in the concentration rather than to absolute concentrations. Thereby even the slightest concentration changes can lead to signal transduction changes (Stryer 1988).
For the very low but constantly present molecules of the intermediaries this is of great functional importance and in general should guide the eye to the effect of antihomotoxic preparations. Here, considerable research demand is necessary.
GPCR Coupled Receptors as Information Amplifiers
The GPCRs belong to the metabotropic receptors, i.e. they form no pores in the cell membrane, but are activated by ligands. These may be available only in the smallest concentrations because the receptors usually block them (Arndt-Schulz Law). Cytokines, like e.g. the colony stimulating factors, have their highest activation potential after the receptor binding with molar concentrations between 10-11 and 10-13 (Contrevas et al. 1991).
The intermediaries of the TCA cycle are available only in micromolecular concentrations (He et al. 2004). The weak signals can be strengthened above all by the Ga-adenylate cyclase-cAMP cascade (Fig. 4). Then every ligand receptor complex catalyzes the formation of a lot of Ga-GTP molecules by which increased cAMP is formed. On the other hand cAMP activated protein kinase can strongly increase the synthesis of target proteins (Stryer 1988, Neves et al. 2002).
The G proteins are involved in the regulation of all cell functions, they have a systemic effect (Fig. 3). For the intermediaries of the TCA cycle a tremendously broad spectrum of activity results from it.
An implication for the whole of homotoxicology results from this, because through this the broad spectrum of activity all homeopathic complex remedies can be informally explained.
In this connection the hormesis effects of antihomotoxica must be discussed (overview by Heine 2006): This has shown itself very well in the pressed juice of Rhus toxicodendron (Rhus tox). The plant juice constitutes the strongest natural contact allergen (overview by Heine 2008). Already from a 100 times dilution or potency (D2) the mother tincture loses this poisonous effect and with a D6 overturns into a clear inflammation inhibition and antigenic-toxicity in relation to tumor cells (Lu et al. 2000, Heine and Andrä 2007, Heine 2011). With other dilutions or potencies these effects disappear again.
Afterwards it was pointed out by Heine (2008) that a Rhus decoction and its anti-tumor effect were generally known since antiquity. The most important pharmacologist of ancient times, Dioscorides (ca. 200 AD) indicated it in his Materia Medica. It was partly used up until the 1950s. How and why was it edged out of the chemotherapy of orthodox medicine? Rhus tox is currently still used as an important pain remedy in homeopathy (Heine 2008). The general inhibition effect of Rhus tox (D6) on tumors was discussed as an inhibition of the tumor marginal inflammation in the growth area (“leading edge”) against normal tissues (Heine 2011). Since apparently all tumor cells have GPCRs available (Entschlagen et al. 2011), the inhibition of the inflammation in the tumor margin might explain a G protein-provided effect.
 An Exclusive Translated Article for Members
An Exclusive Translated Article for Members
From THE BRIDGE Newsletter of OIRF
Published December 2011
From an article in CO’MED, No. 8, 2011
Machine Translation by SYSTRAN, Lernout & Hauspie, LogoMedia & Promt
Translation & redaction by: Carolyn L. Winsor, OIRF
© Copyright 2011, Prof. Dr. Hartmut Heine, Neuhausen, Germany
Recommendations for Further Reading:
- Berg JM, Stryer L, Tymoczko JL. Biochemistry. 7th ed. WH Freeman (United States) 2011
- Calabrese EJ. Hormesis and medicine. Br J Clin Pharmacol 2008; 66: 594-617
- Contreras R, Demolder J, Fiers W. Cloning and expression of cytokine genes. In: Balkwill FR (Ed) New York: Oxford University Press 1991, p.1
- Correa PR, Kruglov EA, Thompson M, et al. Succinat is a paracrine signal for liver damage. J Hepatol 2007; 47: 262-269
- Entschladen F, Zänker KS, Powe DG. Heterotrimeric G protein signaling in cancer cells with regard to metastasis formation. Cell Zyklus 2011; 10: 1086-91
- Farfel Z, Bourne HR, liri T. The expanding spectrum of G protein diseases. N Engl J Med 1999; 340: 1012-1020
- Goldberg ND, Passonneau JV, Lowry OH. Effects of changes in brain metabolism on the levels of citric Zyklus intermediates. J Biol Chem 1966; 241: 3997-4003
- He W, Miao F, Lin D, et al. Citric acid Zyklus intermediates as ligands for orphan G-protein – coupled receptors. Nature 2004; 429: 188-103
- Heine H, Herzberger G, Bauer G. Die Therapie mit intermediären Katalysatoren in der Praxis. 2. Aufl. Baden-Baden: Aurelia-Verlag 2002
- Heine H. Lehrbuch der biologischen Medizin. Stutlgart: Hippokrates 2006; S. 134, 208
- Heine H. Ist Rhus toxicodencron L. (Toxicodendron quercifolium Greene) in homöopathischer Aufbereitung zur adjuvanten Tumorbehandlung geeignet? Ein systemischer Review. Schweiz Zschr GanzheitsMedizin 2008; 20: 35-40
- Heine H. Tumorentzündung und Metastasierung – Hemmung durch Rhus toxicodendron L. (D6). Die Naturheilkunde (im Druck) 2011
- Heine H, Andrä F. Hemmung von Tumor Matrix Metalloproteinasen durch Rhus toxicodendron L. – eine in vitro Studie. Erfahrungsheilkunde 2004; 53: 193-208
- Herbert SC. Physiology: orphan detectors of metabolism. Nature 2004; 429: 143-145
- Hotchkiss RS, Strasser A, McDunn JE, et al. Cell death. N Engl J Med 2009; 361: 1570-1583
- Lu W, Gminski R, Mersch-Sundermann V. Studien zur Antigenotoxizität der Urtinktur und flüssiger Verdünnungen von Toxicodendron quercifolium (HBA 2000) in humanen, metabolisch kompetenten Hepatomzellen: Biol Med 2000; 29: 300-304
- Martin M, Ferrier B, Baverel G. Transport and utilization of alpha-ketoglutarate by the rat kidney in vivo. Pflügers Arch 1989: 413. 217-224
- Melino G. The Sirens’ song. Nature 2001; 412:23
- Neves SR, Prahlad TR, Iyengar R. G protein pathways. Science 2002; 296: 1636-39
- Pajor AM, Sun NN, Joshi AD, Randolph KM. Transmembrane helix 7 in the Na(+)/dicarboxylate co-transporter 1 is an outer helix that contains residues critical for function. Biochim Biophys Acta 2010 (Print-Electronic, EPD 220101110 ISSN 006-3002)
- Pischinger, A.: Das System der Grundregulation. Grundlagen für eine ganzheitsbiologische Theorie für Medizin. 4. Aufl. K. F. Haug Verlag, Heidelberg 1975
- Rattan SIS. Targeting the age-related occurrece, removal, and accumulation of molecular damage by hormesis. Ann NY Acad Sci 2010; 1197: 28-32
- Reckeweg HH. Homotoxikologie. Ganzheitsschau einer Synthese der Medizin. 7. Aufl. Baden-Baden: Aurelia-Verlag; 1993: 618-622
- Rubic T, Lametschwandtner G, Jost S, et al. Triggering the succinate receptor GPCR91 on dendritic cells enhances immunity. Nature Immunology 2008; 9: 1261-69
- Schmid F, Rimpler M, Wemmer U. Antihomotoxische Medizin. Baden-Baden: Aurelia-Verlag 1996
- Stryer L. Biochemistry 3rd ed. New York: WH Freeman, 1988: S.978-985
- Wajant H. The Fas signaling pathway: more than a paradigm. Science 2002; 296: 1635-36
- Zhang DW, Shao J, Lin J. et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 2009; 325: 332-336



