Possibilities for Biological Therapy
By nature, oxygen is a bi-radical and thus is a potential cause of cancer. Already with our first breath death is initiated by the internal oxidation processes. [Theoretically] without oxygen ours would be a life without end. But we must constantly breathe in order to survive. Even a five minute abstention from oxygen is no longer compatible with life.
Thus, absurdly or dangerously, it seems that oxygen is not for our life. Because approximately 1.5 billion years ago anaerobic living cells united with aerobic prokaryotes (mitochondria) into today’s known aerobic eukaryotic cells (endosymbiont theory), in order to gain more oxygen combustion into effective energy.
Oxygen provides a 19-fold higher energy efficiency in the cell
However the biochemical metabolic pathways necessary for it are possible only in the mitochondria. Oxygen thereby functions as an electron acceptor. In order to gain oxidative phosphorylation in the mitochondrial ATP, the cells also need hydrogen as an electron donor. This is provided by the metabolization of our basic food, protein, carbohydrate and fat.
Hypoxic, oxygen poor situations force our cells to switch to the archaic anaerobic way of glycosis (fermentation), to be able to provide further energy. With a passing hypoxia, like with a muscular strain, we know this change over to fermentation is reversible.
If cells retard to the archaic original cell, then they need no more oxygen to live. They become immortal, because the oxygen radicals remain saved in them. However all abilities for a higher life are lost to them. These primitive cells must then again switch over to sugar fermentation in order to survive at all.
Such a development into the archaic happens with the cancer cell. Due to the long lasting hypoxia conditions in the tissue, besides the compulsory decline of the mitochondria resulting from it, increasingly sugar must be transferred into the cell to protect the survival of the cell. Consequently we find the hypoxia inducing transcription factor-1 (HIF-1) increasingly expressed with all growth factors of the glycolysis [Stryer, Biochemie, 2003, 491].
The Warburg Hypothesis, which already postulated these connections more than 80 years ago, was recently scientifically recognized and published in the German Medical Journal in January 2006.
Therefore with cancer diseases we find a clearly increased activity, for example of the hexokinase and the lactate dehydrogenase (LDH) [Cancer Cell, 2006]. Likewise an increased insulin sensitivity of the cancer cell exists with an enhanced glucose absorption through the glucose transporter (GLUT).
In the healthy body cell hydrogen in the mitochondrial respiratory chain phosphorylation is enzymatically (dependent on Vitamin B3 and B2) transferred onto the oxygen protons and electrons by means of cytochrome c (see Figure 1).
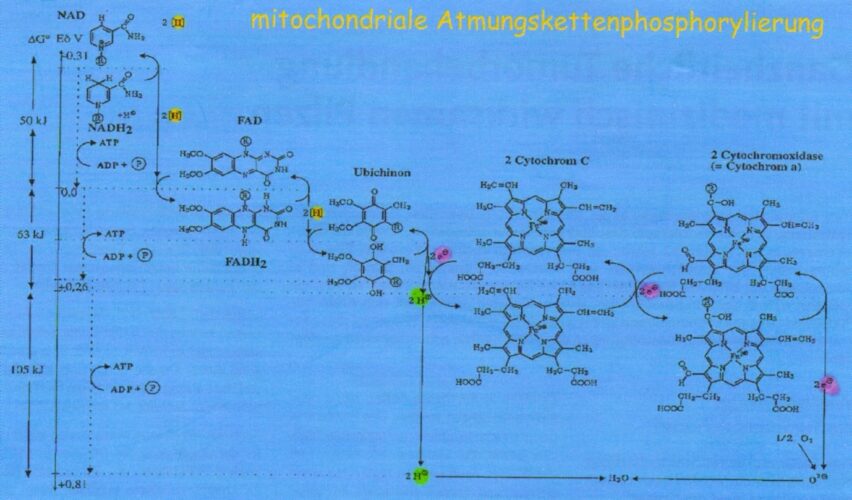
Figure 1: Oxidative phosphorylation (respiratory chain) in the mitochondria
Hypoxia and Anemia
The chronic hypoxia condition of the tissue of a cancer patient leads to a disturbance of the electron transfer from Complex III to IV and therefore with the continuing oxygen deficiency by release of cytochrome c, in the end leads to the destruction of the mitochrondria.
Because cytochrome c causes apoptosis, it is increased through the enzyme Haemoxigenase-1 which reduces the blood group of weakened cytochrome c. Here iron and carbon monoxide (CO) are set free (see Figure 2).
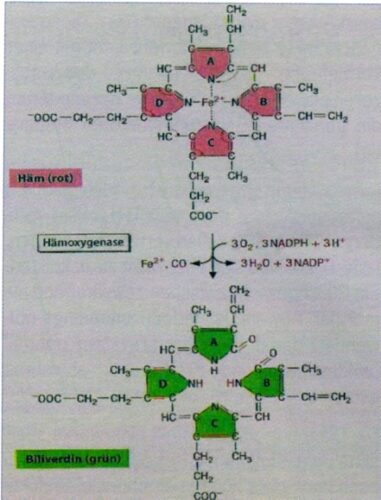
Figure 2: Reduction of cytochrom c by the enzyme Haemoxigenase-1
With the cancer patient Haemoxigenase is over-expressed to prevent the final destruction of the whole cell.
With reinforced degradation of the blood group (whether in the cytochrome c or also the hemoglobin of the erythrocytes) an increased and accumulated bivalent iron is stored mainly in the ferritin – by which the increased ferritin values of cancer patients can be explained. From this tumor anemia can also be derived.
At the same time the freed CO has a 300x higher connection affinity for the erythrocytes than for oxygen, so that the hypoxia is further strengthened with carcinogenesis. Additionally, the CO gas counteracts the nitrogen monoxide (NO) gas which contributes to a further weakening of the killer cells and the Th-1 cells of the immune system [H. Kremer, Barcelona].
Acid is Not Funny
Another substantial problem with cancer patients is the hyper acidification of the tissues resulting from lactic acid (lactate) accumulated from fermentation.
Besides the bicarbonate and hemoglobin buffering, the respiration serves to buffer off the increasingly accumulated protons. Because when breathing we take in oxygen under simultaneous delivery of carbon dioxide. Thus we adjust the pH value in the blood with our breathing.
CO2 + H2O « HCO3 + H+
At the same time as anemia exists additionally the hemoglobin (Hb) buffer system is weakened, which further aggravates the hyper acidification for cancer patients, because 1 mol of Hb buffers off 0.7 mol of protons.
Cancer patients who eat mainly carbohydrate rich, on the basis of the respiratory quotient (RQ = CO2 / O2) buffer out 1.0 of the Hb and only 70% of the protons. Thus sweet makes acid! Therefore over acidified cancer patients should prefer carbohydrate poor nutrition.
With protein combustion the RQ amounts to at least 0.83 and about 90% of the protons can be buffered out.
The RQ with fat oxidation amounts to 0.71 by which all protons can be intercepted. Therefore fat does not lead to hyper acidity.
A Therapeutic Option
Oxygen multi-step therapy increases the arterial pO2 and thus the ATP production. The Na/K-ATPase is activated by the increased ATP supply, sodium and thus also water from the endothelial cells of the arteriole and simultaneously potassium are pumped into the cell. The result is a reduction of the capillary wall edema and an improvement of the blood circulation equally as well as the oxygen supply (See Figure 3) – with all positive results for the cellular change.

Figure 3: Failure of the Na/k-ATPase through oxygen deficiency
Oxygen remains an indispensable elixir of life in spite of its bi-radical character.
 An Exclusive Translated Article for Members
An Exclusive Translated Article for Members
From THE BRIDGE Newsletter of OIRF
Published June 2009
From an article in CO’MED, Nr. 08 / 2007
Machine Translation by SYSTRAN, Lernout & Hauspie, LogoMedia & Promt
Translation & redaction by: Carolyn L. Winsor, OIRF
© Copyright 2007, Dr. Alfons Meyer, Wiesbaden, Germany
Literature:
- Löffler, Petrides, Biochemie und Pathobiochemie, Springer 1998, 711
- Jeremy / Stryer, Biochemie, Spektrum Verlag, 2003, 490ff
- Janeway, Immunologie, Spektrum Verlag 2002
- Kremer, Die stille Revolution der Krebs- und AIDS-Medizin, Ehlers Verlag, 2004
- Voet, Biochemie, VCH Verlag, 1994, 538
- Beyermann, Chemie für Mediziner, Thieme Verlag, 1979, 46ff
- Klinke / Silbernagel, Lehrbuch der Physiologie, Thieme Verlag, 2000, 243
- Ardenne, Spektrum Verlag, 1996, 157ff
- Rehner / Daniel, Biochemie der Ernährung, Spektrum Verlag, 2002, 24ff



